Website: Snowflakes and Snow Crystals
 The SnowCrystals Website is maintained by Kenneth Libbrecht, chairman of the Physics Department at Caltech, who describes it as being “all about snow crystals and snowflakes — what they are, where they come from, and just how these remarkably complex and beautiful structures are created, quite literally, out of thin air.
The SnowCrystals Website is maintained by Kenneth Libbrecht, chairman of the Physics Department at Caltech, who describes it as being “all about snow crystals and snowflakes — what they are, where they come from, and just how these remarkably complex and beautiful structures are created, quite literally, out of thin air.
On it, visitors can learn about the physics of snowflakes, how to make ice spikes in their freezers, the creation of synthetic snowflakes at Caltech, and see stunning images of snowflakes captured by Libbrecht using a photomicroscope. Site visitors can also purchase “The Secret Life of a Snowflake,” a book aimed at readers ages 6 to 12.
Be sure to explore the sidebar, which contains more specific information on snowflakes and ice — crystal faceting, snowflake branching, ice properties, “myth and nonesense” — as well as a guide on photographing snow.
The morphology diagram, below, demonstrates how the shape of a snowflake depends on the temperature and humidity:
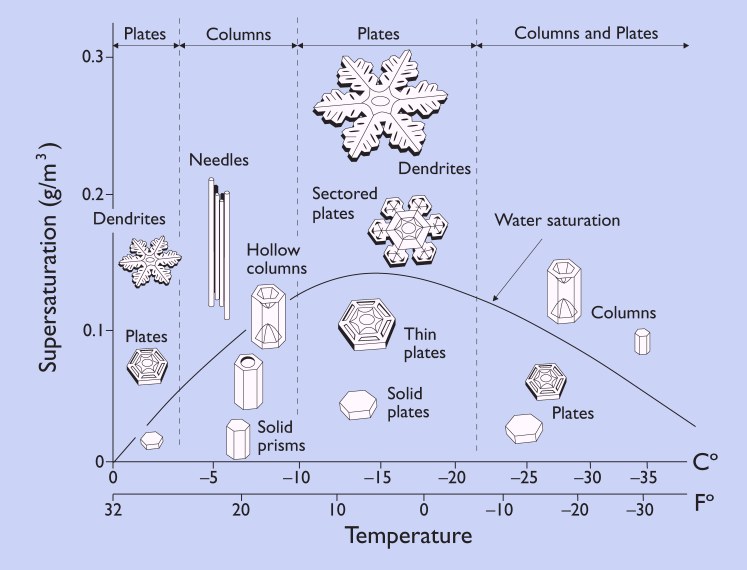 From SnowCrystals.com: The morphology diagram tells us a great deal about what kinds of snow crystals form under what conditions. For example, we see that thin plates and stars grow around -2 C (28 F), while columns and slender needles appear near -5 C (23 F). Plates and stars again form near -15 C (5 F), and a combination of plates and columns are made around -30 C (-22 F).
From SnowCrystals.com: The morphology diagram tells us a great deal about what kinds of snow crystals form under what conditions. For example, we see that thin plates and stars grow around -2 C (28 F), while columns and slender needles appear near -5 C (23 F). Plates and stars again form near -15 C (5 F), and a combination of plates and columns are made around -30 C (-22 F).
 Furthermore, we see from the diagram that snow crystals tend to form simpler shapes when the humidity (supersaturation) is low, while more complex shapes at higher humidities. The most extreme shapes — long needles around -5C and large, thin plates around -15C — form when the humidity is especially high.
Furthermore, we see from the diagram that snow crystals tend to form simpler shapes when the humidity (supersaturation) is low, while more complex shapes at higher humidities. The most extreme shapes — long needles around -5C and large, thin plates around -15C — form when the humidity is especially high.
Why snow crystal shapes change so much with temperature remains something of a scientific mystery. The growth depends on exactly how water vapor molecules are incorporated into the growing ice crystal, and the physics behind this is complex and not well understood. It is the subject of current research in my lab and elsewhere.
Filed under: Web Resources
Tags: Physics, Science of Snow, Winter








