Activity: Propel a Toy Boat with Chemicals

(Modified activity drawn from the University of California Irvine Chemistry Outreach Program, Science IDEAS, and CSIRO)
Level: 4-12. Time Required: 20-30 minutes. This activity can also be used as a 5-minute demonstration.
PROPEL A TOY BOAT WITH CHEMICALS
In this lesson, students learn about the concept of cohesive forces in water that contribute to surface tension; how much force it takes to break water’s surface; and how a simple chemical — liquid soap — can be used to part the water molecules. The activity demonstrates how to make use of the surface tension of water, not just to float a toy boat, but to make it move.
Materials
- A long, shallow tray or sink, filled with water
- Scissors
- Thick cardboard or thin plastic (like the lid of a margarine container)
- A toothpick, pencil or eye dropper
- A few drops of liquid detergent or flakes of dry detergent
Introduction
Have you ever wondered how it is possible for a water spider to stay afloat on the surface of a pond? The answer is found in a concept called surface tension, which is related to how much force it takes to break the water’s surface. A small bug doesn’t have enough weight to move the water molecules apart, and so it can actually stand on the water’s surface.
Engineers develop chemicals that can disperse the molecules of liquids to help remove pollutants from our water and air. In light of the disastrous 2010 BP oil spill, there is a great deal of discussion — and concern — about the use of chemical dispersants that could help dilute the oil in the Gulf of Mexico but might also harm the surrounding environment and wildlife. What are dispersants, how do they work, and what are the dangers associated with them?
Read more about the properties and uses of dispersants on the ITOPF (International Tanker Owners Pollution Federation) Website
Read about the use of Dawn detergent in cleaning oil off coastal wildlife suffering from the 2010 spill (Washington Post, June 17, 2010).
Activity
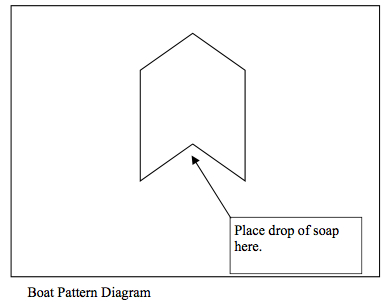
1. Have each student or small group of students draw a simple boat on a piece of card, like the one shown in the figure. Caution students to cut carefully to produce a triangular section at the rear that lines up with the front tip of the boat.
2. Fill a large bowl or sink with water and place the boat in the middle of it to make sure it floats well.
3. Next, take a little detergent and drop it carefully in the rear triangular section behind the stern of the boat. Just a drop will do — to be control the amount, use an eye dropper, toothpick, or pencil to dispense the liquid. Observe and record what happens to the boat. Is it being pushed or pulled? (Answer: pulled — see the explanation below).
Note for teachers: within a few seconds, students’ boats will travel forward through the water. If the boat is cut evenly, it will travel forward in a straight line.
After testing one or two boats, you will need to refresh the water, as its surface tension will be reduced by the detergent, and no longer pull the boat well.
 What happened? Understanding the reaction:
What happened? Understanding the reaction:
It looks as though the boat is being pushed along by the detergent. But, in fact, it is being pulled by the water in front of it.
In many liquids, the molecules of the liquid are attracted to each other. This attraction, or cohesive force, makes the surface of the liquid act like a stretched-out balloon skin. Any point on the surface of a liquid is under tension, and different liquids have different amounts of surface tension. In water, the tension is only very slight and it is fairly easy to break through the surface. But if you have ever done a ‘belly-flop’ into a swimming pool, you have felt the effect of surface tension.
When the paper cutout boat is first placed in the tray of water, adhesive forces between the water molecules and the paper pull the cutout equally in all directions. The result is that all of the forces balance out and the paper does not move (much!)
When the detergent is added at the back of the boat, the surface tension and the water’s adhesive properties are reduced. With no more adhesion, the water in the rear area stops pulling on the paper. The water in front of the cutout still has its adhesive properties, so it continues to pull on the boat, making the boat move forward. The triangular notch helps the boat drag a little bit of the detergent with it, so it travels further.
Extensions
Discussion Questions:
1. Why does the boat move forward only when the soap touches the water?
2. What made the boat be pulled forward?
3. What did the soap or detergent do to the cohesive forces between the water molecules?
4. What other shapes of boats would work the same way?
5. Would it work without a triangular space in the boat?
6. What would happen if we touched the soap to the side of the boat?
Activities:
- Try repeating this experiment with a drop of oil from an oil can or a small piece of camphor. They both change the surface tension of the water.
- Challenge students to redesign their boats to improve performance, then test the new designs, and make further design refinements.
- Teachers located near a beach can conduct this sand-castle building experiment from the National Park Service that further demonstrates the cohesive properties of water.
Further Study:
Assign older students to investigate the use of chemical dispersants in tackling water pollution, and the associated challenges of their use. Students can research current news stories, such as a filter solution proposed for the 2010 oil spill proposed by Di Gao, a chemical and petroleum engineering professor.
Filed under: Class Activities, Grades 6-8, Grades 9-12, Grades K-5
Tags: Chemistry, Grades 4-12, Lesson Plan