Activity: Taking the Heat
(Provided Courtesy of TeachEngineering and the Integrated Teaching and Learning Program, College of Engineering, University of Colorado at Boulder)
Ever touch a metal object on a hot day? The heat burns your fingers. But the ground is cool enough to walk on barefoot. Different materials have a different heat capacity, something engineers consider in designing everything from houses to hair dryers. In this activity for grades 3-5, students compare the heat capacity of different materials and learn why it is an important property of thermal energy. They work in groups of three, using thermometers. Time required: 50 minutes.

Engineering Connection
For safety, engineers must understand the heat capacity of insulators and conductors. They wear rubber gloves, as an
insulator, when measuring volts and amps through a metal wire with a low-heat capacity. When designing any electrical system (computers, buildings, motors, etc.), engineers keep heat capacity in mind to avoid causing short circuits in the equipment or shocking people who perform equipment maintenance.
Engineers who design passive solar heating systems take advantage of the natural heat capacity characteristics of materials. They build into structures some thermal mass, usually made from a high-heat capacity material such as masonry, concrete or water that can store the sun’s heat energy for an extended time and release it slowly after the sun sets, to prevent rapid temperature fluctuations. Engineers also consider heat capacity and thermal energy when they design food containers and household appliances.
Standards :
See how this lesson plan fits national and state standards by clicking here and scrolling down to Educational Standards.
Learning Objectives
After this activity, students should be able to:
- Define heat capacity.
- Explain how heat capacity determines a material’s ability to store thermal energy.
- Measure the temperature of a material over time.
- Describe the difference between materials with high-heat capacity and low-heat capacity.
- Understand that materials with high-heat capacities store thermal energy better than materials with low-heat capacities.
- Determine which material has the highest heat capacity and, thus, stores thermal energy well.
- Explain why engineers need to know about the heat capacity of materials when designing equipment, structures and products.
Materials
Each group needs:
- 2 thermometers
- 1 250-300 ml beaker or small jar
- 1 container to hold small beakers/jars (cake pans or larger jars work well)
How Much Heat Will It Hold? Worksheet (pdf), one per student
For the entire class to share:
Any of the following materials, a different one for each group: sand, water, shredded paper, shredded Styrofoam, cloth, or powdered materials such as Sakrete (concrete), plaster or finely crushed rock. Students can also bring from home specific materials the teacher approves for testing.
- Hot water (~85°C), enough for each group to fill large jars
- Ice water, enough for each group to fill large jars
- 2 large beakers, cans, dishpans or other containers (for cold and hot water control setup)
- Measuring cups (or jars with 200 ml level marked)
- Masking tape
- Markers
Introduction/Motivation
Today, we are talking about thermal energy or heat energy. Have you ever been outside on the playground on a sunny day and touched the metal of a swing set? How does it feel? It is hot? Yes! How about walking barefoot on a sunny day? Have you ever walked on the sidewalk and had to jump to the grass because the pavement was too hot for your feet? Do you think the grass and the pavement are actually different temperatures — even if it is the same temperature outside? They probably are not different temperatures at all!
Different objects require different amounts of heat to raise the same amount of material to the same temperature. You can notice this on a hot summer day when the ground is cool enough to walk on, but the road and sidewalk are very hot, or a metal bench is much hotter than a wooden bench. The metal bench requires less heat to make it hot than the wooden bench. When we measure this property, the quantity is called the heat capacity of he material. When an object absorbs heat, the thermal energy is spread among the atoms and molecules in the material. Energy makes the molecules vibrate back and forth. If the vibrations become faster, we measure it as an increase in temperature. Every material has a different heat capacity.
Another way of explaining a materials’ heat capacity is to think about it as the measurement of thermal energy storage, just like temperature is the measurement of thermal energy given off. Heat capacity is how much thermal energy a material stores up and temperature is how much thermal energy a material gives off. Today, we are going to look at the heat capacity of some different materials.
Engineers use their knowledge of the thermal properties of matter to design everything from engines to satellites to houses. They use a material’s heat capacity to determine its usefulness for different applications. A material with a low-heat capacity (such as metals) has a greater increase in temperature from absorbing the same amount of heat as a material with a high-heat capacity (such as water). This is why materials with high-heat capacities, such as water, are used for storing thermal energy.
Other materials with high-heat capacity, such as brick or concrete walls, are important to engineers designing housesthat they want to stay warm in cold climates. Engineers consider heat capacity when working with any material. For example, think of all the devices and appliances in your house. If the wiring in your computer or lamp or hair dryer gets too hot, it may spark and stop working.
Vocabulary/Definitions
- Heat: A form of energy associated with the motion of atoms or molecules, and capable of being transmitted through solid and fluid media by conduction, through fluid media by convection, and through empty space by radiation.
- Heat capacity: The amount of heat required to raise the temperature of one mole or one gram of a substance by one degree Celsius without a change of phase (from solid to liquid, or liquid to gas, etc.).
- Thermal energy: The energy an object has due to the motion of its particles. Also called heat energy.
- Thermometer: An instrument for measuring temperature, especially one having a graduated glass tube with a bulb containing a liquid (typically mercury or colored alcohol) that expands and rises in the tube as the temperature increases.
Procedure
Before the Activity
Calculate the quantity of each material that you will need in order to provide each student group with ~4/5 cup (200 ml) of one of the materials. Gather materials and make copies of the How Much Heat Will It Hold? worksheet.
Set up stations for each test material: container of material, thermometers, jars, measuring cups or jars with 200 ml level marked and bucket (or other container for used materials).
Prepare enough ice and hot water for the class. Wait until right before starting the activity to put hot water in one bucket. Put the ice and tap water in another bucket for the second half of the activity.
As a control, the teacher measures the temperature of the hot water and ice water during the 10 minutes. This data may be used as comparison at the end of the activity, during a discussion of water temperature heat loss to the room air. The students evaluate the following variables: 1) The kind(s) of materials they are testing, and 2) Water temperature (testing in hot water or cold water or both).
With the Students
- Divide the class into student teams of three or four students each.
- Assign team “jobs.” In each team, have one student in charge of reading the temperature on the thermometer in the material, another to read the temperature in the water and a third to be the recorder. If you have any groups of four, have the fourth student be in charge of watching the time between measurements.
- Ask the students to predict which material will have the highest heat capacity (store thermal energy the best), and record this on their worksheets.
- Have each team go to the appropriate station and look at their materials. Instruct them to measure 200 ml of their assigned material using a measuring cup or jar with the 200 ml level marked.
- Have the student testing water pour the hot water in his/her container (large jar) and place a thermometer in the container. Have students testing the powdered material, sand or dirt, fill the beaker halfway (small jar), place a thermometer into the material and then finish filling their containers. The material must cover the thermometer bulb or bottom.
- Using masking tape, label each container.
- Measure and record the initial temperatures of the materials and water. Remind the recorders to document the starting temperatures on their worksheets.
- Place the beakers/jars of material into the large container of hot water.
- Have students measure and record the temperature of the material and the water bath every minute for 10 minutes (see Table 1 for a sample data table). Record the starting temperature of the 200 ml beaker of water, sand, and paper, etc., and the temperature of the hot water bath at “0” minutes. Record the temperature reading of each of the thermometers every 1 (or 2) minutes in the table. Record the temperature reading of each of the thermometers every 1 (or 2) minutes in the data table.
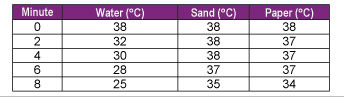
- Sample data table of temperature readings of different materials in an ice bath.

- Have each team member pour the contents of their beaker/jar into waste containers at the appropriate station.
- Repeat the entire activity using ice water instead of hot water.
- After all measurements have been made, direct students to make a bar graph showing their material and the highest temperature or the lowest temperature it reached.
Conclude with a class discussion:
Compare the results of the different materials tested. Compare graphs. Which materials had the highest heat capacity (stored heat the longest)? Also compare the temperature gains (losses) to the teacher’s measurement of the hot water and ice water, taken as a control. Discuss the water’s heat loss (and gain) to (and from) the air in the room.
Ask students to comment on their initial predictions, as recorded on the worksheets. What have they learned? Ask them to explain their understanding of which materials have a high-heat capacity and which have a low-heat capacity.
How would an engineer use what the students have learned today? Which of the materials that the class measured would an engineer choose for insulating a home in the winter? To design a good food storage container for soup? For the design of a product that you want to heat up quickly?
Troubleshooting Tips
Caution students to pour dense materials, like sand, powdered concrete or dirt, into the container first, place the thermometer in the container and then continue filling it.
Students should not force a thermometer into a dense material because the thermometer might break.
Some materials, like sand (after being used) need to be collected in a separate container until they return to room temperature, since they may be cold or warm for a while as a result of the experiment. Dense materials gain and lose heat slowly. To obtain the correct amount of materials, students can estimate the 200 ml of sand or other materials in a 250 ml beaker.
Obtain the hot water from the tap (around 85°C) or the teacher can heat a larger container of water (85°C) and distribute the water to students.
Assessment
Pre-Activity Assessment
Prediction: Have the students predict which material will have the best thermal energy storage (or, hold in heat the longest) and record their predictions on the worksheets.
Activity Embedded Assessment
Worksheet: Have students follow along with the activity and record measurements on the How Much Heat Will It Hold? worksheet. After students have completed their worksheet, have them compare answers with their peers. Review their answers to gauge their mastery of the subject.
Graphing: Have students graph the time vs. temperature results of their particular material on their worksheet.
Post-Activity Assessment
Closing Discussion: The teacher should measure the temperature of the hot water and ice water over the 10 minutes as a control. This can be used as comparison at the end during a discussion of water temperature heat loss to the air in the room.
Prediction Analysis: Have students compare their initial predictions with their test results, as recorded on the worksheets. Ask the students to explain their understanding of which materials have a high-heat capacity and which have a low-heat capacity.
Engineering Analysis: Have student s compare their graphs to other teams’ graphs and determine which material had the highest heat capacity (stored heat the longest). Which of the materials that the class measured would an engineer choose for insulating a home in the winter? (Answer: The ones with high thermal storage.) Which of the materials would an engineer use to design a good food storage container for soup? (Answer: One with a high thermal energy storage capacity.) Which of the materials would an engineer use for the design of a product that you want to heat up quickly? (Answer: Something with a low thermal storage capacity.)
Activity Extensions
- If time, direct students to either record the number of minutes each material took to lose a two degree increase in temperature after the heating occurred, or to record how long each material took to gain two degrees in temperature after being cooled.
- Have the students complete the activity again, bringing in other odd materials from home. Create a class list ranking the heat capacity of different materials from greatest to least.
- Have students make a thermos using a small jar inside a large jar with one of the materials from this activity as the buffer around the small jar. Have the students place hot or ice water inside their thermos and measure the temperature in regular intervals to see how effective the thermos is.
Activity Scaling
For advanced students, create a combined class chart of all the data for each test material. Have the students make a line graph to show the temperature change for each material when placed in a hot bath and when placed in an ice water bath. Which one had the highest heat capacity (stored heat the longest)?
This lesson can be found on the TeachEngineering website, where it is titled, “How Much Heat Will It Hold.”
References
Testing Thermal Storage Materials Lesson Plan. Renewable Energy Lesson Plans, Infinite Power, Texas State Energy
Conservation Office. Accessed October 5, 2005. (Source of activity) http://www.infinitepower.org/lessonplans.htm
Contributors: Sabre Duren, Jeff Lyng, Malinda Schaefer Zarske, Denise Carlson
©2005 by Regents of the University of Colorado.
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0226322. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Filed under: Class Activities, Lesson Plans








