Let the Blood Flow: Biomedical Artery Unclogging Experiment
TeachEngineering activity contributed by Science and Engineering of the Environment of Los Angeles (SEE-LA) GK-12 Program, UCLA. For pre- or companion activity, see TeachEngineering’s Clearing a Path to the Heart (grades 6 – 8) and Blood Clots, Polymers, and Strokes and The Mighty Heart dissection (grades 9-12). For a longer capstone + writing project for high school students, see Artificial Heart Design Challenge
Summary
In this NGSS-aligned activity, high school students work as biomedical engineers to find liquid solutions that can clear away polyvinyl acetate polymer “blood clots” in model arteries made of clear, flexible tubing. Teams create samples of the “blood clot” polymer to discover the concentration of the model clot and then test a variety of liquids to determine which most effectively breaks it down. Students learn the importance of the testing phase in the engineering design process, because they are only given one chance to present the team’s solution and apply it to the model blood clot.
Engineering Connection
Biomedical engineers apply their understanding of scientific and mathematical principles to create practical solutions to biological problems. The highly interdisciplinary field includes diverse applications of new technologies created to improve patient treatment. With circulatory system diseases a leading cause of death in the U.S., engineers have designed, tested and implemented many devices, such as artificial hearts, artificial valves, stents, as well as medical and surgical procedures, to save lives. In the case of life-threatening blood clots in blood vessels, biomedical engineers research the biochemistry and structure of blood clots and apply this knowledge to develop and test methods for harmlessly breaking them down.
Prerequisite Knowledge
Familiarity with laboratory techniques and safety.
Learning Objectives
After this activity, students should be able to:
- Explain the importance of the testing stage in the engineering design process before presenting and using a solution to a problem.
- Describe methods and approaches biomedical engineers may use to develop solutions to dangerous and life-threatening blood clots in blood vessels.
- Apply their understanding of the formation and degradation of polymers to develop liquid solutions that break down polyvinyl acetate polymers.
Related Activities
- Develop models to describe the atomic composition of simple molecules and extended structures. [Grades 6-8]
- Communicate scientific and technical information about why the molecular-level structure is important in the functioning of designed materials. [Grades 9-12]
- Construct and revise an explanation based on evidence for how carbon, hydrogen, and oxygen from sugar molecules may combine with other elements to form amino acids and/or other large carbon-based molecules. [Grades 9-12]
Common Core Mathematics Standards
- Use volume formulas for cylinders, pyramids, cones, and spheres to solve problems. [Grades 9-12]
International Technology and Engineering Educators Association
Materials
Each group needs:
- 4 paper cups
- 4 wooden stirrers
- clear, flexible tubing (3/4-inch diameter X 5/8-inch interior diameter X 4-inch length)
- rubber stopper, a size that temporarily fits and blocks the tubing
- white glue, 60 ml
- 1 cup (~237 ml) of 4% borax solution (50 ml)
- graduated cylinder (50 ml)
- water
- marker, for labeling the paper cups
- 1 cup (~237 ml) of 1 M HCl (hydrochloric acid), such as a 1 liter bottle for $9.75 at http://Carolina.com/
- 1 cup (~237 ml) of 1 M NaOH (sodium hydroxide), such as a 1 liter bottle for $12 at http//Carolina.com/; alternatively, see note below
- 1 cup (~237 ml) of enzyme solution, such as item #15666 for $36 at Valley Vet (4-ounce Zymox Otic with 1% hydrocortisone enzymatic solution that contains three active enzymes); alternatively, see note below
- 1 cup (~237 ml) of NaCl solution (make in bulk for the class by adding 1 cup table salt to 1 gallon water and stirring rapidly until the salt is dissolved)
- 1 cup (~237 ml) of glucose solution (make in bulk for the class by adding 1 cup granulated sugar to 1 gallon water and stirring rapidly until the sugar is dissolved)
- 1 cup (~237 ml) liquid dish or laundry detergent
- 6 test tubes
- 6 droppers or pipettes
- safety goggles, one per student
- lab apron, one per student
- gloves, one pair per student
- Let the Blood Flow Student Handout, one per student
Note: The enzyme solution and the sodium hydroxide solutions could be replaced with less expensive alternatives. Only the hydrochloric acid (HCl) solution is a viable solution in this activity, thus, the other solutions (sodium hydroxide, enzyme solution, NaCl solution, glucose solution, liquid detergent) are provided so students have testing options for experimentation, ultimately discovering that they do not work. For example, for the purposes of this activity, since the enzyme solution is so expensive, replace it with any other inexpensive, readily accessible and unidentifiable liquid, perhaps an unfamiliar juice (cabbage juice?) or even Windex, and call it the enzyme solution.
To share with the entire class:
- hazardous waste bin, to collect polymer waste material
- bucket, to collect any spilled liquid during testing
- dish soap, for cleaning test tubes
Introduction/Motivation
(Have ready a model blood clot in an artery, made with polymer-blocked clear tubing, as described in the Procedures section.)
Some biomedical engineers and scientists focus their research and creativity on developing therapeutic methods to unblock human blood vessels that might lead to ischemic stroke. Investigators typically conduct experiments with models before testing their treatments on humans. (Show students the model blood clot in tubing).
In this activity, your goal is to find a method to unclog the tubing without using mechanical force. Just as biomedical engineers first studied the blood clotting process before developing therapies, you will first experiment with the polymerization of the white clog in the tube.
The white clog is made by mixing polyvinyl acetate (C4H6O2)n with borax (sodium borate, Na2B4O710H20), to form a rubbery white polymer. Polyvinyl acetate is a large polymer made of vinyl acetate monomers covalently bound together:
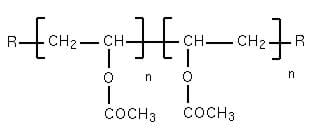
In the presence of water, borax forms the following borate-boric acid buffer system:

When mixed together, the polyvinyl acetate is cross-linked, trapping water, and produces the white clog:
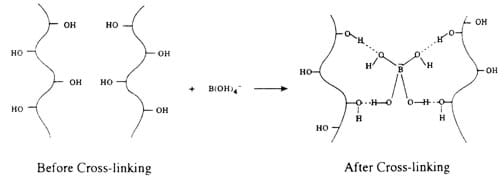
Diagrams copyright: Azim Laiwalla, UCLA SEE-LA GK-12 Program
In this lab, your objective is to explore chemical treatments that are able to degrade the white clog and restore flow in the tube.
Procedure
Before the Activity—Teacher
- Gather materials and make copies of the Let the Blood Flow Student Handout.
- Prepare for each group one model blood clot in an artery (clear tubing obstructed with a polymer “clot”), following these steps:
- Place the stopper one inch from the bottom of the four-inch length of clear, flexible tubing.
- Prepare the polyvinyl acetate polymer by mixing together 15 ml white glue, 30 ml water, 10 ml 4% borax solution and stirring well.
- Quickly pour the polymer into the tubing, about one-half inch in height.
- Remove the stopper from the tubing.
Before the Activity—With the Students
- Review with students the content presented in the associated Blood Clot, Polymers and Stroke lesson. Assign students to answer the pre-lab questions provided in the Assessment section (page 1 of the student handout). See answers HERE.
Biomedical engineers help to save lives in the case of potentially deadly blood clots of “thrombus” in the blood vessels.
copyright: Agency for U.S. Healthcare, Department of Health and Human Services
With the Students—Instructions
Divide the class into groups of five students each.
Direct students to follow the instructions on the student handout to make four polyvinyl acetate polymers of varying concentrations. Remind them to record all observations—color, texture, viscosity, smell, etc. Direct them to make polymers in the paper cups only—do not pour white glue in the graduated cylinders. Below is a teacher version of the instructions on the student handout:
- Label the four paper cups as polymers 1, 2, 3, 4.
- Fill the graduated cylinder with 15 ml of water and pour into paper cup 1. Mark the level of the water in the cup. Pour out the water. Mark the same level on the three other cups.
- For each paper cup trial, fill the white glue up to the line on the paper cup. Vary the amounts of borax solution and water based on the table in the student handout.
- Stir well and once polymer is formed, knead for several minutes and continue to observe and record observations in the table. (As necessary, guide students to discover the correct polymer by comparing their samples to the model “blood clot” in the “artery.”) Based on your observations, determine which one of the polymers created is the same concentration as the “blood clot” in the artery. (Answer: Polymer 2.)
- Develop a liquid solution to unclog the artery (tube).
- Design an experiment using your knowledge of making polymers and its molecular structure to test how well each of the six liquids (1 M HCl, 1 M NaOH, enzyme solution, 5% NaCl solution, glucose solution, liquid detergent) break down the polyvinyl acetate polymer. Make sure the correct concentration of the polyvinyl acetate polymer is being used for the tests; if necessary, create more of this sample for testing.
- An example experiment: Add a small sample of the polymer to the test tube, and then a sample of one of the liquids. Begin with a few drops of the liquid. If you do not see anything happen, add a few more drops. Record your observations on the handout. Empty the test tube contents into the hazardous waste bin and clean out the test tube.
- Repeat the previous step for the remaining five liquids.
- After testing is done, present and demonstrate your method to the entire class. Describe to the class the steps your group took to discover your best liquid solution. Over a bucket, pour your liquid solution into the model artery to the class how effective it is at breaking down the “blood clot.”
- Have students prepare lab reports with the following sections: objective, hypothesis, materials and methods, data, data analysis, discussion and conclusion.
- Assign students to answer the post-lab questions by the day after the activity.
Worksheets and Attachments
Safety Issues
- Students must wear protective equipment, including chemical goggles, lab apron and gloves.
- Make sure the classroom or lab used for this activity has lab safety equipment, including a safety shower, first-aid kit, telephone, fire extinguisher, eye wash and fire blanket.
- Do not allow students to put the polymer in their mouths.
- Refer to the following Carolina Biological Supply Co. website for specific information about handling hydrochloric acid (HCl) and sodium hydroxide (NaOH) on Carolina’s activities and resources website for teachers.
Troubleshooting Tips
Students may feel that they do not have enough instruction to scientifically find a liquid solution to unclog the tube and rather decide to just test all available solutions—urge students to use their knowledge of how the polymer forms on a molecular level.
Investigating Questions
Based on the molecular properties of the polymer and the chemical equilibrium of borax in water, what agent would degrade the white clog? (Answer: The hydrochloric acid degrades the polymer [plaque]. When the polymer [plaque] solution is created, the reaction between B(OH)3 and 2 H2O molecules forms B(OH)4– and H3O+ in a reversible manner. By adding acid, H3O+, the equilibrium shifts to the left or towards the reactants, thereby degrading the polymer. All solutions except the hydrochloric acid are essentially red herrings—expect them to do nothing to the model clot!)
Assessment
Pre-Activity Assessment
Pre-Lab Questions: On the day prior to conducting the activity, have students answer the following questions, as provided on page 1 of the Let the Blood Flow Student Handout. See answers in the Let the Blood Flow Student Handout Example Answers. Review answers with students and briefly explain the blood clotting procedure.
- What is a polymer? How does a polymer form? What type of atom most commonly allows the formation of natural polymers?
- Is a blood clot a polymer? Is it a protein?
- What are causes of stroke?
- Flow rate is the amount of fluid that flows per unit time: f= Av, where f is the flow rate, A is the cross-sectional area of the pipe/vessel, and v is the speed of the flow. What is the flow rate of blood through a vessel that is 1 cm in diameter moving at 5 cm/s?
- If the same blood vessel as in question 4 has a blood clot that is 2 cm long and 0.7 cm thick, what is its estimated volume in centimeters? What is the new flow rate? What is the difference from your previous answer?
Activity Embedded Assessment
In-Class Presentation: Have teams present their biomedical methods and solutions to the entire class, explaining why they chose that liquid solution, what concentration they used, and what they think will happen. Conduct the final liquid solution trials in front of the entire class. Ask students to suggest ways to improve their results. In addition, ask students to explain how the molecular structure of the polymer makes it clog the tubing. (Hint: remind students of the reactions discussed in the Introduction/Motivation.) Have them explain the properties of the polymer that might make it useful for other purposes.
Post-Activity Assessment
Lab Report: Have students complete lab write-ups with the following sections: objective, hypothesis, materials and methods, data, data analysis, discussion and conclusion. Review their lab reports to gauge their comprehension.
Post-Lab Questions: As homework, assign students to answer the post-lab questions on the student handout by the day after the activity. Review their answers to gauge their comprehension.
- If a blood vessel was clogged with a polyvinyl acetate polymer, would the solution your group found be applicable? Explain.
- If you were developing a cure or treatment for patients with life-threatening blood clots, what would you need to do before trying it on the actual patients?
References and Additional Sources
Chemistry. Encyclopædia Britannica. 2009. Encyclopædia Britannica Online.
Heart Disease and Stroke Statistics. 2009 Update: A Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. 2009.
Video: Atherosclerosis. National Library of Medicine MedLine resource
Contributors
Azim Laiwalla, Ann McCabe, Carleigh Samson
Supporting Program
Science and Engineering of the Environment of Los Angeles (SEE-LA) GK-12 Program, UCLA
This digital library content was developed by the University of California’s SEE-LA GK-12 program under National Science Foundation grant number DGE 0742410. However, these contents do not necessarily represent the policies of the National Science Foundation, and you should not assume endorsement by the federal government.
Filed under: Class Activities, Grades 9-12, Lesson Plans
Tags: arteries, Biomedical Engineering, Chemistry, circulatory system, Class Activities, Grades 9-12, heart, Lesson Plan, NGSS, stent, TeachEngineeirng, UCLA SEEK GK-12 program, valves