Lesson: Hydrogen — Electrolysis of Water
(Lesson Courtesy Florida Solar Energy Center). Level: Students in grades 6-8. Time Required: One hour. Lesson Pdf.
High-energy Hydrogen I: Electrolysis of Water
This lesson engages students in grades 6-8 in an activity to help them understand how hydrogen is created and then extracted from water, and used as an energy source.
Student Objectives
- to be able to explain how hydrogen can be extracted from water
- to be able to explain how energy flows through the electrolysis system
Standards:
Florida Sunshine Standards Benchmarks/Grade Level Expectations
Benchmark SC.A.1.3.5 – The student knows the difference between a physical change in a substance and a chemical change.
Sixth Grade Level Expectations:
- knows the chemical properties of various substances
- knows the difference between a physical and chemical change
Seventh Grade Level Expectations:
- knows that chemical changes result in new substances with different characteristics
Benchmark SC.B.1.3.1 – The student identifies forms of energy and explains that they can be measured and compared.
Sixth Grade Level Expectations:
- understands that energy can be converted from one form to another
Eighth Grade Level Expectations:
- knows examples of natural and man-made systems in which energy is transferred from one form to another.
Benchmark SC.B.1.3.2 – The student knows that energy cannot be created or destroyed, but only changed from one form to another.
Sixth Grade Level Expectations:
- understands that energy can be changed in form
Eighth Grade Level Expectations:
- understands how the principle of conservation of energy is applied during an energy transfer.
Benchmark SC.B.1.3.4 – The student knows that energy conversions are never 100% efficient.
Seventh Grade Level Expectations:
- knows that useful energy is lost as heat energy in every energy conversion
Eighth Grade Level Expectations:
- knows that energy conversions are never 100% efficient and that some energy is transformed to heat and is unavailable for further useful work
- knows that a transfer of thermal energy occurs in chemical reactions.
Benchmark SC.G.2.3.1 – The student knows that some resources are renewable and others are nonrenewable.
Sixth Grade Level Expectations:
- knows renewable and nonrenewable resources
Eighth Grade Level Expectations:
- knows that some resources are renewable and others are nonrenewable.
Materials:
- photovoltaic cell (3V min) or 9-volt battery (1 per group)
- piece of aluminum foil, approx. 6 cm x 10 cm (2 per group)
- sodium chloride
- electrical wires with alligator clips (2 per group)
- beaker larger than 250 ml (1 per group)
- water
- stirring rod or spoon
- graduated cylinder (several per class)
- Digital mulitimeter, with ranges of 0-2 [DCV and 0-20 DCV] (1 per group)
- test tubes (2 per group)
Background Information
Key Words and Definitions:
compound – composed of two or more substances, ingredients, elements, or parts
electrolysis – chemical change, especially decomposition, produced in an electrolyte by an electric current.
electrolyte – a compound decomposable, or subjected to decomposition, by an electric current
element – a substance composed of atoms having an identical number of protons in each nucleus; elements cannot be reduced to simpler substances by normal chemical means.
hydrogen – A colorless, highly flammable gaseous element, the lightest of all gases and the most abundant element in the universe.
molecule – the smallest part of a substance that retains the chemical and physical properties of the substance and is composed of two or more atoms.
oxygen – an element that at standard temperature and pressure is colorless, tasteless, and odorless (required for nearly all combustion and in the cellular functioning of animals)
Background:
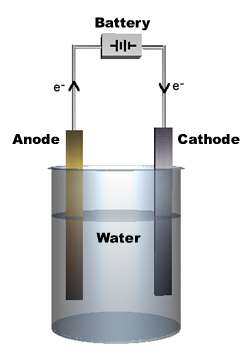
When you add salt to the water, the salt ions (which are highly polar) help pull the water molecules apart into ions. Each part of the water molecule has a charge. The OH- ion is negative, and the H+ ion is positive. This solution in water forms an electrolyte, allowing current to flow when a voltage is applied. The H+ ions, called cations, move toward the cathode (negative electrode), and the OH- ions, called anions, move toward the anode (positive electrode).
At the anode, water is oxidized: 2H O T O + 4H+ + 4e- 22
At the cathode, water is reduced: 4HO+4e- T2H +4OH- 22
Note that there is a net balance of electrons in the water. Bubbles of oxygen gas (O2) form at the anode, and bubbles of hydrogen gas (H2) form at the cathode. The bubbles are easily seen. Twice as much hydrogen gas is produced as oxygen gas.
The net reaction: 2H2O T 2H2 + O2
Procedure
1. Read the following passage to the students (this passage also appears on the student’s science journal):
Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. By adding electricity to a liquid and providing a path for the different particles to follow, a liquid such as water can be separated into hydrogen and oxygen.
Explain that in this experiment, they will be taking a sample of salt water and adding a flow of electricity to it (electrolysis). They will see the hydrogen and oxygen bubbling up.
2. Divide the students into lab groups of 3 – 4 students per group. Distribute the directions for this lab from the Student Journal (download student pages from Lesson Pdf). They are to follow the directions, perform the lab, and complete the questions in their journals.
Student journal material:
Questions
1. What is it that you are trying to accomplish in this experiment?
2. State a few facts that you have learned about electrolysis and it uses.
3. Write a hypothesis of what you think might happen during your experiment.
Directions
- Accordion-fold each piece of aluminum foil down the long way so that you have two pieces approximately 1 cm x 6 cm. These are going to be your electrodes.
- Press each electrode flat.
- Bend the top 1 cm of each electrode over to act as a hanger. They will be hung on the inside of your bowl.
- Attach one end of each wire to the hanger of your electrode with a paper clip.
- Dissolve salt into water at the ratio of one teaspoon salt for each 50 ml of water. Stir to dissolve the salt.
- Hang the electrodes on the inside of the bowl so that they hang down into the water. They should hang a couple inches apart; do not let them touch during the experiment. Add more salt water if necessary.
- Attach the other end of each wire to your photovoltaic panel or a battery. Make a note which electrode is attached to the positive and which is attached to the negative.
- If using photovoltaics, take your electrolysis device outside into the sun.
- Record your observations below.
Observations
- What did you see happening at the positive electrode?
- What did you see happening at the negative electrode?
- Explain in your own words what is occurring in your electrolysis system?
- Imagine that electrolysis and hydrogen are the areas that you would like to do research in for science fair. What variables and conditions in the experiment that you just did could you change or vary to find out what happens?
- What would your hypothesis be?
Evaluation
Lead a discussion on what the students observed and the significance of their
observations. Points to cover may include:
- Electrolysis produced a chemical change (contrast to a physical change if this hasn’t been taught yet);
- The chemical formula for water is H2O = two atoms of hydrogen to every atom of oxygen;
- the energy flow in this system: Energy cannot be created or destroyed….so where did the electrical energy go? (Answer: The electrical energy is transformed into chemical energy that splits the molecules apart.);
- If you left this apparatus working long enough, it would heat up the water. Why? (Answer: No energy conversion is 100% efficient–some energy is transformed to heat);
- The process can be reversed using a fuel cell–hydrogen and oxygen are combined to make electricity and water;
- Hydrogen is used as a combustible fuel on the Space Shuttle and can also be used to make electricity with a fuel cell
- hydrogen is a renewable resource, and is non-polluting when used as an energy source
Extensions
1. After successfully performing and evaluate this experiment, students can address other issues, such as determining:
a. How varying the concentration of the salt water affects the number of bubbles produced by the electric current.
b. How varying the amount of electricity in the hydrolysis circuit affects the amount of gas being produced.
c. How they could capture and measure the amount of gas produced.
2. Assign students to research where hydrogen currently being used in the United States and in their own state; how much is used per year; and where is it produced. How does the research and production of hydrogen in their state help the economy? The environment?
3. Have students research, then write a one-page definition of a hydrogen fuel cell and how it works.
Helpful Internet Sites
http://www.energyquest.ca.gov/projects/index.html
Activities and projects for students in K-12, including a number of electricity projects.
http://www.cheminst.ca/ncw/experiments/eelectrowater.html
A Website with information about electrolysis including the chemical reaction that takes place.
Filed under: Grades 6-8, Lesson Plans
Tags: Electrical, Electrical Engineering, Lesson Plan, Water